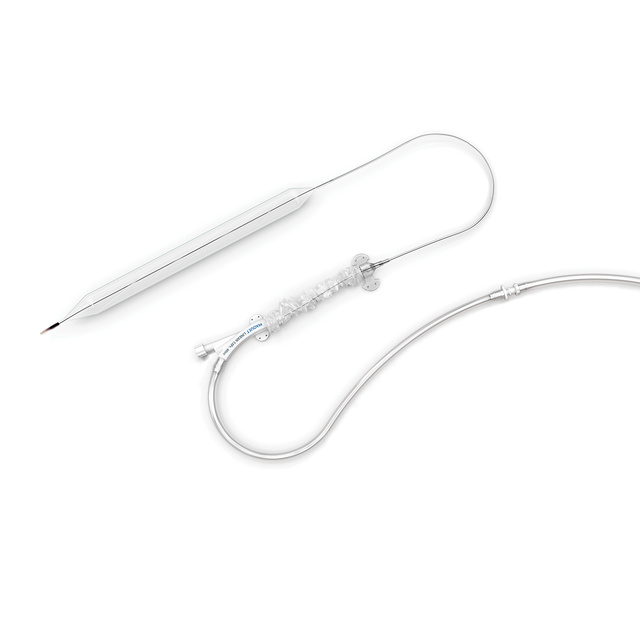
Improved Sheath
- Improved Sheath to Introducer Transition
- Hemostasis Valve
- Twist Lock Introducer Hub
- Universal Color Coding for Size
Potential For Reduced Complications*
All balloon and catheter materials meet FDA and ISO biocompatibility guidelines for devices and materials. LINEAR 7.5 Fr. IAB catheters and insertion kits do not contain latex.
* Bench testing completed by Maquet. Data on file. Bench test results are not necessarily predictive of clinical results.
Ideal for small adults, diabetics, and patients with PVD*
- Reduced catheter cross-section area by 12% vs. 8 Fr.
- Smaller catheter size has potential to increase distal limb flow
- Minimized size of arterial puncture
Proprietary IAB membrane provides better performance*
- Abrasion resistance improved by 43%
- Immediate inflation at start-up
- Improved fatigue resistance
Faster inflation cycle times
- Faster gas shuttle performance allows support of rapid heart rates and arrhythmias
- 19% faster cycle time in 80° bend test method when compared to competitor
T-Handle protector for balloon membrane
- Keeps the membrane tightly wrapped prior to insertion
- Helps to handle catheter prior to insertion
* Comparison of Outcomes After 8 vs. 9.5 French Size Intra-Aortic Balloon Counterpulsation Catheters Based on 9,332 Patients in the Prospective Benchmark® Registry. Catheterization and Cardiovascular Interventions 56:200-206 (2002).
Visit our Academy – training and education designed to enhance your proficiency
For more information about our onsite events or remote trainings, you can also contact your local sales & service representative.