High sterilization throughput in pharma industry
- Flexible, modular design to enable sterilizer customization
- ECO process to minimize energy consumption
- Minimized risk of drug recall due to lack of sterility assurance
- Innovative load carriers for large units prepared for automated solutions
- Future-proof control system that is prepared for easy integration in your platform

Ideal for Component and Terminal Sterilization
Components sterilization
For porous goods that are often packed in small and medium sizes, like filters, textiles, rubber stoppers in bags or other wrapped items, the GTS enables indirect pre-heat, drying and cooling through its fan system. Sterilization is fast and efficient.
Terminal sterilization
For liquids packaged in open containers like open/vented glass bottles or liquids packaged in closed containers like glass ampoules, glass bottles/vials, syringes, BFS containers, or plastics bottles/bags, the GTS enables steam/air mix ventilator sterilization cycles as these require terminal sterilization by heat. Quality, safety and efficacy of these product in mostly large quantities will not be affected.
Scalability in chamber size
The GTS steam/air mix sterilizer will come with a small, medium or large chamber* - whatever fits your production space best
The modular design enables you to efficiently design your sterilization process. By adding or removing sections from the various zones, you can increase or decrease your sterilization capacity.
* small and medium size will be available for purchase in 2025
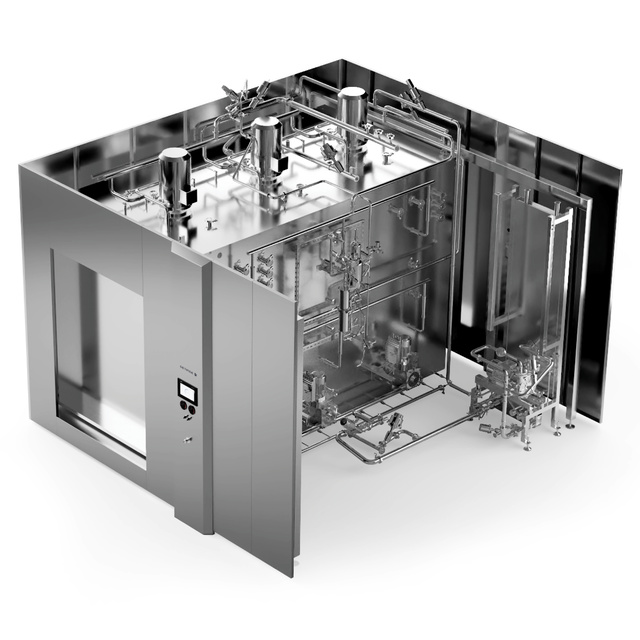
The GTS explained
The GTS explained
The GTS sterilizer is designed for terminal air/steam sterilization of liquid products in flexible and rigid containers that require rapid heating and cooling with the demand for a dry load after the process. The sterilizer is specifically designed for sterilization of product/combinations that are pressure sensitive such as syringes, flexible plastic or rigid glass containers.
Available in small, medium and large to match your cleanroom requirements
Pressure and temperature sensors within the chamber control and adjust when necessary
The GTS offers three control systems: B&R, Siemens or Allen Bradley.
The ergonomic display has an intuitive HMI with presets for several common processes, making it easy to use and ensures operator safety and a reproducible process– as well as product integrity.
Program parameters can be adjusted to the specific process needs, depending on the application
The fully automated Getinge Roller Conveyor System (GRCS) is designed for loading and unloading shelf and rack-laden batches of pharmaceutical products into and out of GTS sterilizers. It removes the need for human operators to risk injury or repetitive strain.

Future-proof cleanroom performances with SIMATIC WINCC Unified
Open and flexible visualization system for both cleaning and sterilization in the biopharma industry. Excellent operator control and monitoring with SIMATIC HMI, integrated in both Getinge cGMP washers and sterilizers. The new generation washers and sterilizers come with the latest open interface, SIMATIC WinCC Unified, to meet the challenges of digitalization in the pharmaceutical industry. The SIMATIC HMI Unified Panels allow for collaboration between Getinge cGMP washers and sterilizers to reduce engineering effort and to seamlessly integrate usability.
Pressure and temperature control
GTS Steam/Air Mixture Sterilizers use sterile compressed air to apply support pressure to balance the environment with the internal load pressure, meeting each load’s requirements for heating and cooling rates. Pressure and temperature sensors within the chamber control and adjust when necessary. A fan and ducting create a circulation pattern and maintain a homogeneous environment when it comes to temperature and pressure.
Flexible process design for various applications
Optionally, program combinations are available for steam sterilization of equipment, instruments, utensils, liquids in vented containers, glassware, filters, rubber material.
Program parameters can be adjusted to the specific process needs. If there are specific requirements, cycles can be customized to fulfill requirements of the application.
Modular configuration with zones
Due to its modular design with zones, you can configure the GTS sterilizer to match your requirements, varying from small, to medium to large sterilizers.
Getinge's patented system allows the zones of the steam/air mixture sterilizer to be independently controlled, so a single process can be validated for either full or part batches.
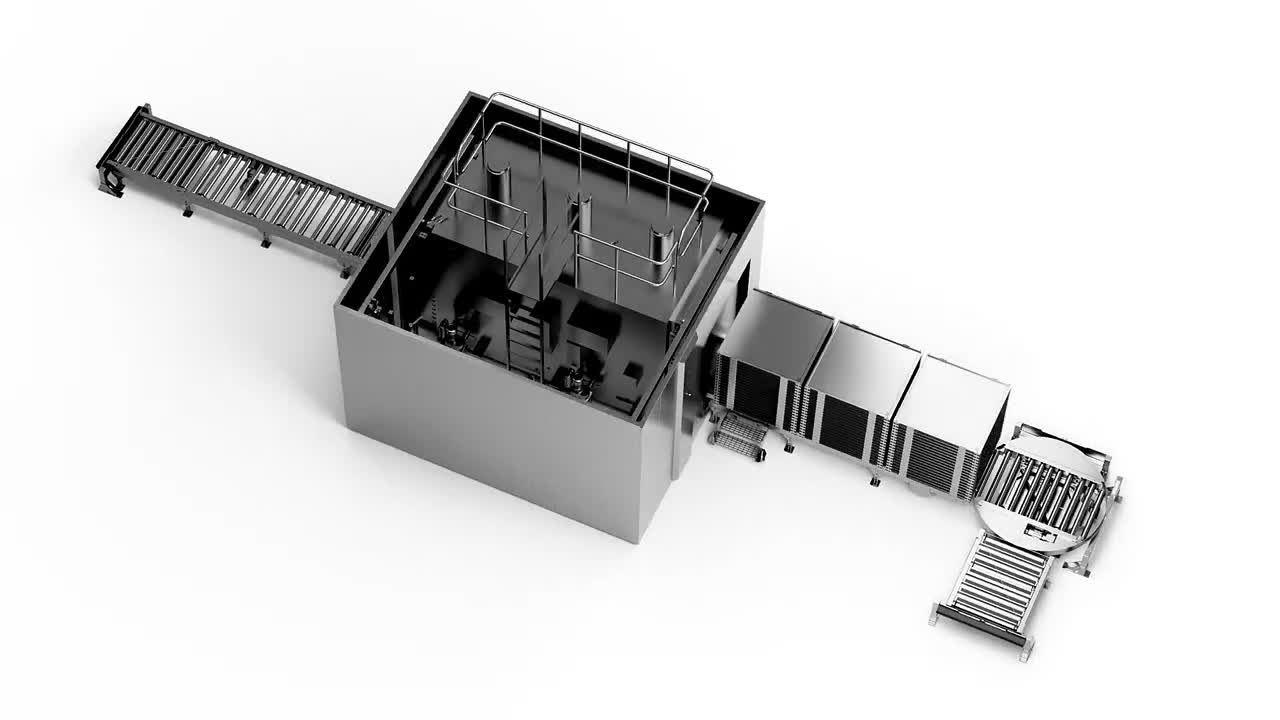
The modular, automated load handling solution for the Getinge GTS sterilizer
The Getinge Roller Conveyor System (GRCS)
The GRCS ensures safe, continuous pharmaceutical production by optimizing load-handling performance throughout the terminal sterilization cycle.
- Optimizes production efficiency, uptime, and throughput
- Protects the GTS sterilizer from loading and unloading risks
- Eliminates risk of human injury
- Prevents heavy or unwieldy loads being dropped
- Support traceability for quality assurance and compliance
- Compliant with applicable safety standards
- Easy-to-use operations
- Modular design makes it suitable for all production environments
- Harmonious integration with GTS sterilizers as part of a single-vendor solution
Loading
The GRCS is based around a motorized roller carriageway onto which batches can be placed before safe transfer into the GTS sterilization chamber. This makes the process of placing batches onto the GRCS independent of the process of feeding batches into the sterilizer.
Unloading
Once the terminal sterilization cycle is complete, a corresponding GRCS carriageway at the opposite end of the sterilizer can be utilized to support the unloading process. Each GRCS carriageway can be configured to connect with existing upstream and downstream conveyor systems where required.
Traceability
The automated features of the GRCS include sensors and scanners that are seamlessly integrated with the operation of the GTS sterilizer as part of a complete Getinge solution. This allows full traceability of loading racks and enables autonomous chamber loading and start of sterilization process.
White Papers
-
Explore the validated steam sterilization process for Alternating Tangential Flow devices
-
Discover which sterilization process is compatible and reliable for prefilled syringes
-
Discover how surface roughness and material choice affect cleanability, corrosion resistance and microbial control.
-
Getinge's statement of compliance of its GMP pharmaceutical washers and sterilizers controlled by WinCC Unified software to 21 CFR Part 11 and Annex 11 standards.
-
Discover how Getinge's Life Science equipment supports secure operation, in relation to the key elements of industrial cybersecurity.