Aragen's Validation Journey with Getinge's SUPR Single-Use Bioreactors
Aragen Bioscience, located in Morgan Hill, California, is the biologics discovery and early development subsidiary of Aragen Life Sciences. The Morgan Hill campus supports cell line development (CLD), process development (PD), bioproduction, antibody discovery, and analytical characterization of therapeutic and diagnostic antibodies and other biologics for human and animal health.
In its work, Aragen uses Getinge’s Applikon 2 L, 5 L, and 10 L glass stirred-tank (multi-use) bioreactors to support their client research and development (R&D) programs. Larger-scale production runs needed for preclinical efficacy and toxicology studies were not feasible. As a trial, Getinge offered 50 L and 250 L single-use production reactors (SUPRs) that would allow Aragen to produce larger volumes for preclinical production.
Getinge’s SUPRs are targeted to any production where 50 L or 250 L is a normal production run. The 50 L and 250 L SUPRs increase efficiency, eliminating the clean-in-place (CIP) and sterilization-in-place (SIP), while significantly reducing the risk of contamination. They are GMP compliant, which is useful for companies working on clinical trials, where larger quantities of cells are needed quickly.
Aragen’s goal for this study was to see if their production process — developed using their Getinge Applikon 2 L, 5 L, and 10 L glass stirred-tank reactors (STRs) — could be easily scaled up to larger, single-use bioreactors. Getinge was interested in obtaining customer data for the 50 L and 250 L SUPRs. Thus, Aragen’s partnership with Getinge was born.
Validation Study
During this study, Chinese hamster ovary (CHO) cells were cultured at a production scale of 30 L and 120 L to evaluate the scale-up process. Using their current bioreactor configuration of 2 L, 7 L, and 15 L glass stirred-tank reactors (STR), Aragen scaled up the production to a 50 L SUPR and a 250 L SUPR.
One of the concerns in this study was that the plastic film from the SUPR might release extractable and leachable substances (E&Ls) during the production process. E&Ls may detrimentally affect cell growth or productivity. Adequate cell growth, comparable productivity, and product quality also were expected for a proper scale-up and tech transfer. A variety of process engineering parameters were considered critical, such as the power input/volume ratio (P/V), impeller tip speed, constant mixing time, oxygen transfer rate (OTR), hydrodynamic shear, and bubble formation. For proper cell culture scale-up, it is very important to select bioreactors of different sizes with similar volumetric mass transfer.
Because it is glass, the STR does not leach compounds during the cell growing process as the polymeric plastic bag in the SUPRs might. Previous data analysis of Getinge in-house testing for E&Ls showed that none of SUPR’s E&L compounds affects cell growth. In this study, Aragen wanted to make sure that the plastic of the 50 L and 250 L SUPRs did not affect the cell growth.
In addition, when scaling from a small size to a large size bioreactor, mixing behavior is different and mixing takes longer. Poor mixing can allow the formation of pockets with greater or lesser amounts of nutrients that affect the growth cycle. During mixing, cells also can create byproducts, some of which are toxic. When large pockets of toxins develop, they can kill off large numbers of cells.
Figure 1: Comparison of the growth behavior: (a) viable cell density and (b) viability of cells
Getinge’s 50 L SUPR single-use bioreactor and Getinge’s 2 L Applikon Bio Stirring Glass Bioreactor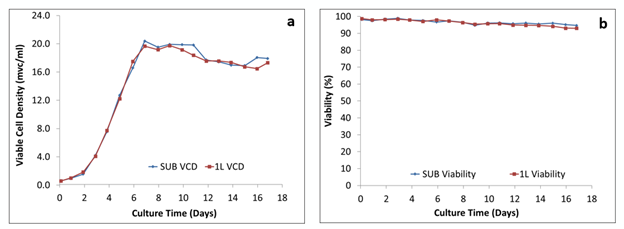
Getinge’s 250 L SUPR single-use bioreactor and Getinge’s 2 L Applikon Bio Stirring Glass Bioreactor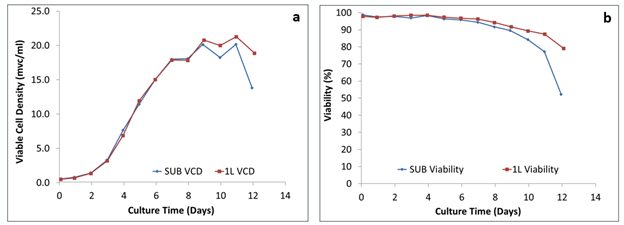
Figure 1 shows that the growth profile, with viable cell density plotted against time as well as the percent viability plotted against time, for both the 50 L and 250 L SUPR mirrored that of the 2 L glass STR, which were run side-by-side. A similar growth pattern was achieved in both.
Figure 2: Comparison of the metabolism profiles: (c) L-glutamine, (d) glutamate, (e) glucose, (F) lactate, and (g) ammonium
Getinge’s 50 L SUPR single-use bioreactor and Getinge’s 2 L Applikon Bio Stirring Glass Bioreactor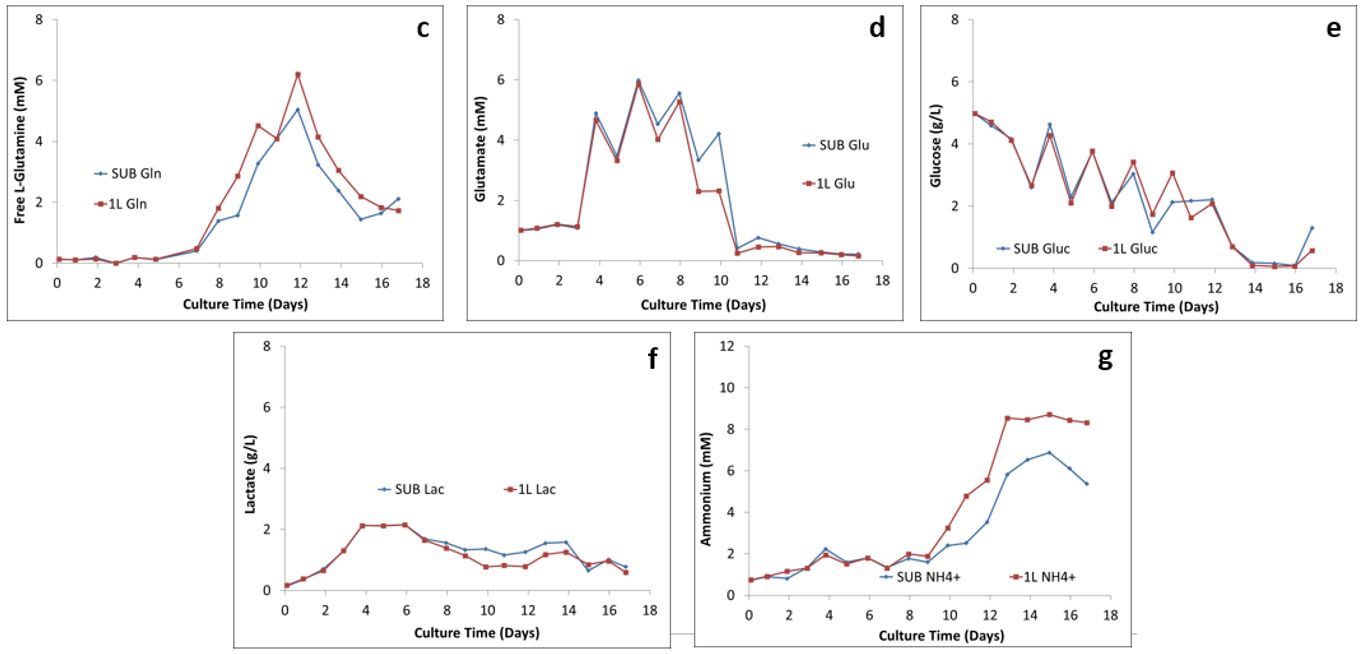
Getinge’s 250 L SUPR single-use bioreactor and Getinge’s 2 L Applikon Bio Stirring Glass Bioreactor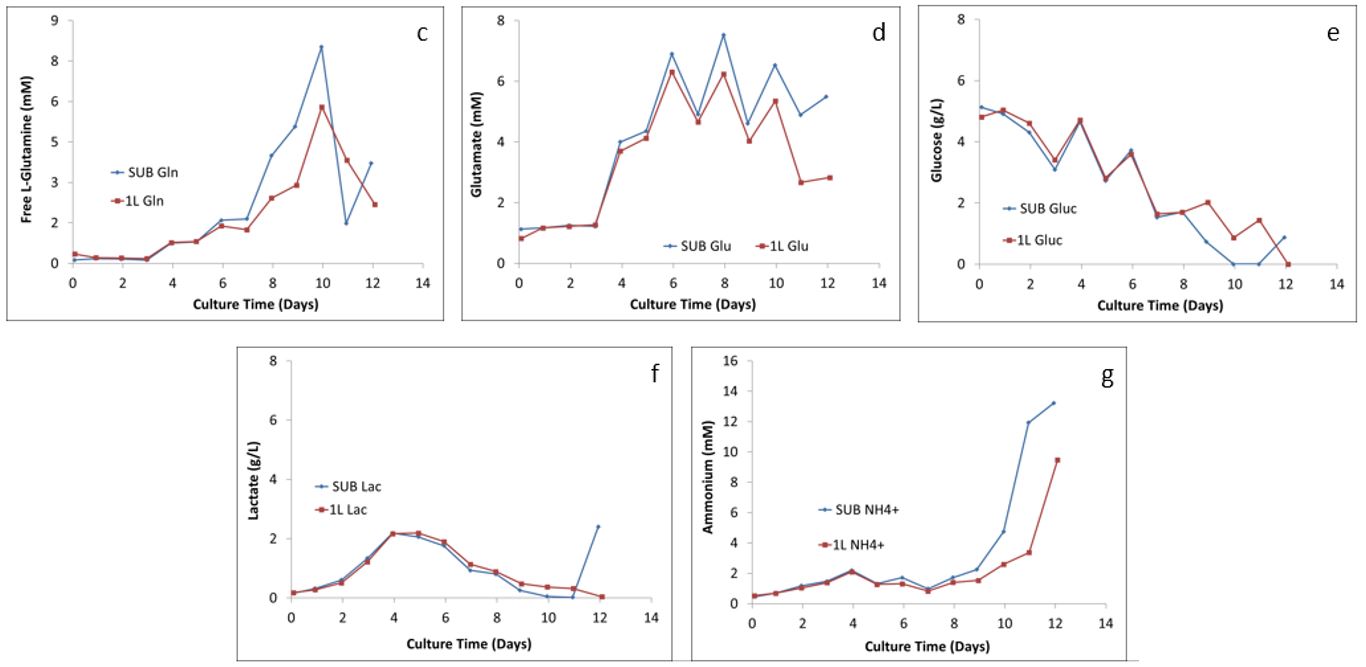
The major metabolic pathway, including byproduct formation (lactate and ammonium) and glucose consumption, in both the 2 L STR and 50 L and 250 L SUPR are shown in Figure 2. As Getinge Senior Application & Cultivation Specialist Marlous van Dijk explained, “You don’t want all the nutrients going to a product you don’t want.” Figure 2 shows that the ratios of the five metabolites created in the SUPRs closely follow the ratios created in the smaller glass STRs, which shows a comparable process performance across the scale.
Figure 3: Comparison titer profile between the 50 L and 250 L SUPR and the 2 L STR
Getinge’s 50 L / 250 L SUPR single-use bioreactor and Getinge’s 2 L Applikon Bio Stirring Glass Bioreactor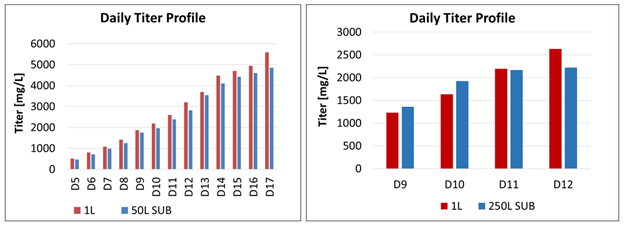
Finally, the daily titer profiles, comparing the 50 L SUPR to the 2 L STR and the 250 L SUPR to the 2 L STR, are very similar (Figure 3). “The end result is good,” van Dijk said. “The difference is within the expected productivity range” and was slightly better than Aragen expected, since some productivity is always lost when scaling up, she said.
Collaborative Approach
Aragen has a lot of experience in scaling up at a small production scale. In this study, they worked collaboratively with the Getinge team to diagnose and troubleshoot the problems in the move to a larger scale-up. Aragen’s model fed-batch process was successfully scaled up from a 2 L STR to 50 L and 250 L SUPRs. “With Aragen, they are experts in process and scaling. They have done scaling at a smaller scale,” van Dijk said. “With a customer with less experience, we [the Getinge team] can share our experience in scaling.”
“The collaboration was very successful,” said Dai Quach, senior scientist at Aragen. “We can talk right away if we diagnose a problem. We learned from each other,” he said. Getinge has “a lot of very good technical people that can support during the troubleshoot.”
Customer Testimonial:
Single-Use Production Reactor (SUPR)
While they found that the transition from bench scale up to pilot or production scale was very challenging to ensure an ideal growth at all scales, setting up the SUPR was no challenge, according to Quach. The setup is a very straightforward and simple installation. The SUPR’s click-and-go bag loading streamlines the process. “We haven’t had any problems. You can recognize all the connectors; you cannot mess it up,” he explained. Both the SUPR 50 L and 250 L proved to be very easy to use, especially, it’s easy disposal.
The bag is ready to use. We don't have to worry about contamination. With the SUPR, we don't worry about anything.
The SUPR geometries and ratios of impeller diameter to vessel inner diameter, and the ratio of maximum liquid height to vessel diameter, are similar to the Applikon glass STRs bioreactors, making scaling up simpler. “Scaling up is easy because the dimensions are similar and the process is the same,” van Dijk explained. “You still have to use a scaling strategy, but you can use a simple strategy with ‘rule of thumb’ calculations.”
Quach feels that makes them economical because the lab doesn’t have to invest in large-scale stainless-steel bioreactors that must be cleaned and sterilized, and the 50 L and 250 L SUPR can be placed anywhere there are gas lines and oxygen to run them. “You don’t have to spend a lot of operating capital or make a lot of capital investments for clean-in-place and sterilization-in-place,” he said.
Because they are so easy to use, Quach said the SUPRs also save on labor costs. A single person can set up a SUPR, where it takes several people to set up a multi-use bioreactor of the same size. “The bag is ready to use,” Quach explained. “We don’t have to worry about contamination. With the SUPR, we don’t worry about anything.”
Quach and the Aragen team were happy with their results. “The present work systematically evaluates the scale-up issues within the scope of mixing, oxygen transfer, and carbon dioxide removal. Everyone in the team shares the same positive feedback. The point is that Getinge has a good product so we can make it work!”

