How Isolators, RABS, Laminar Flow Hoods, and Biosafety Cabinets Compare
During sterile manufacturing, human operators pose the greatest risk for product contamination. To reduce microbiological and particulate contamination risks, aseptic techniques typically rely on either laminar flow equipment used within a cleanroom environment or barrier technology. Laminar flow equipment includes laminar flow hoods and biosafety cabinets (Class I, Class II, and Class III). Barrier technology includes restricted access barrier systems (RABS) and isolators.
Laminar flow equipment including laminar flow hoods (LFH) and Class I and II biosafety cabinets (BSCs) are open systems that rely on uniform, HEPA-filtered air directed across the work surface to physically reduce external particulate contamination. Because they are open systems, they are required to be used within a cleanroom environment. While Class III BSCs are a closed system, they are used primarily for work with highly infectious microbiological agents. Their highly complex operation and high expense make them impractical for use in most aseptic processing techniques.
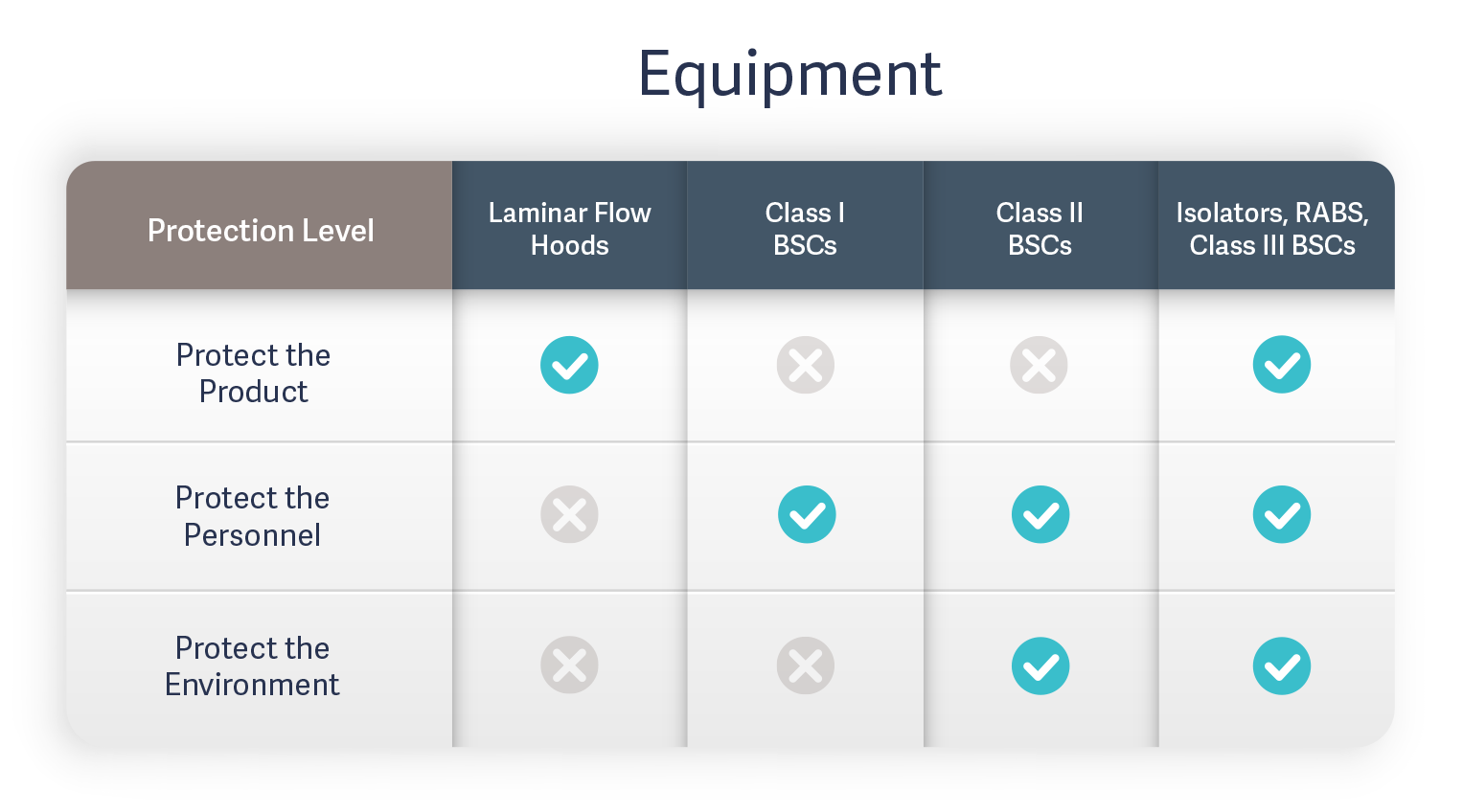
Isolators provide a completely closed system that entirely separates the operator from the process. The hermetic barrier between the product, process, and personnel effectively eliminates the risk of external microbial and particulate contamination while also shielding operators from toxic substances or potent active pharmaceutical ingredients (APIs). This increases product quality control and operator safety, reduces downtime, and makes it easier to trace sources of contamination detected in sterility testing.
Restricted Access Barrier Systems (RABS) come in open and closed configurations and either active or passive types. Open-door RABS provide only a partial barrier. Passive-type RABS do not include an internal HEPA filtration system and instead rely on laminar airflow systems in the production room itself. Active-type RABS include an independent laminar flow system. Typically, both active and passive open RABS must be located within a cleanroom as they rely on the HEPA filtration system in an aseptic space. Only closed RABS operate similarly to isolator technology as they provide a complete barrier and therefore have a lower risk of contamination.
Sterility Assurance in Aseptic Processing
It is crucial that aseptic processing, small batch fill and finish, pharmaceutical compounding, sterility testing and quality control, and other aseptic processing techniques take place under strict aseptic conditions.
Recent EU recommendations in the release of GMP Annex 1 show that the use of BSCs, laminar flow hoods, and cleanrooms is no longer the preferred method for contamination control in sterile product manufacturing. The more stringent requirements of Annex 1 push instead for the use of barrier technology including closed-door RABS or isolators. More pharmaceutical manufacturers are turning to barrier technology for sterility assurance as a result of the increasingly stringent Annex 1 recommendations and the growing demand for liquid biopharmaceuticals, personalized medicines from cell and gene therapy, and mRNA vaccines.
How to Choose Between a Closed-Door RABS or an Isolator for Aseptic Processing?
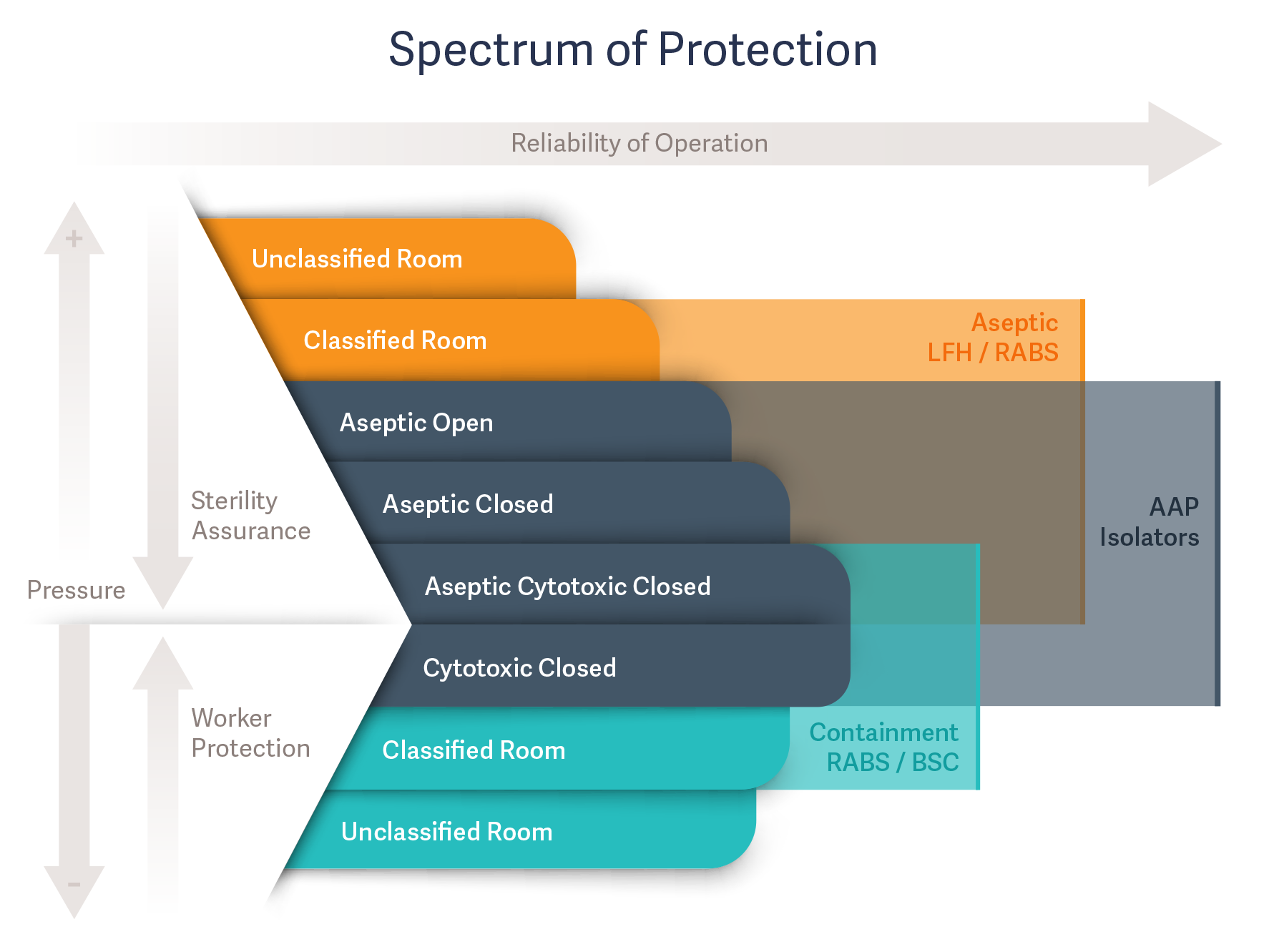
In aseptic processing, a spectrum of protection spans product sterility assurance and worker protection. Selecting the most appropriate equipment depends on the level of protection needed. Examining some of the key areas in which the two systems differ will make it easier to determine which system is best for your unique aseptic process. While closed-door RABS and isolators may seem very similar at first glance, there are some major differences.
One of the main differences between isolators and restricted access barrier systems is that the latter is a broad term that is used for a variety of systems, rather than a standard piece of equipment like an isolator. To be considered a RABS, according to the definition outlined by the International Society for Pharmaceutical Engineering (ISPE), a containment system must have rigid wall construction with glove port access, when necessary, unidirectional airflow, and meet ISO Class 5 standards. Despite this definition, however, it is common for restricted access barrier systems to vary greatly. Because of this, isolators remain the most widely accepted barrier technology used in aseptic processing and product fill operations.
Another key difference between RABS and isolators is the decontamination method. Both open-door and closed-door RABS require manual bio-decontamination. Isolators, however, commonly include automated bio-decontamination cycles, often using H2O2 vapor or a similar sanitizing agent depending on protocols. Automated bio-decontamination provides consistent results that can be easily validated.
Select the Right Isolator and Configuration
Isolators provide greater mitigation against microbial and particulate contamination by containing the process within the chamber and eliminating contact with the operator. This makes it easier to trace sources of contamination, improves sterility assurance, reduces downtime, and provides greater control over the process. They also provide protection from toxic chemicals, drugs, manufactured substances, and potent or highly potent active pharmaceutical ingredients (APIs and HPAPIs) for operators and pharmacists.
Isolators are an acceptable advanced aseptic processing (AAP) method that is ideal for ultra-clean production demands. AAP techniques are generally complete barrier methods used during critical process steps. Laminar flow hoods, biosafety cabinets, and open-door RABS are not appropriate in AAP applications. In addition to complete separation between the process and the operator, AAP techniques require complete ingress control to ensure the highest level of particulate and microbial contamination control. While both RABS and isolators are often used in AAP techniques, the consistency of automated bio-decontamination cycles in isolators gives them a clear edge in terms of contamination mitigation.
Common isolator configurations include those designed specifically for testing procedures and isolators optimized for all common aseptic applications. Getinge isolators are compatible with our full range of DPTE® transfer solutions to ensure safe and efficient transfer without breaking containment.
Getinge Isolators |
||
|
Optimized for sterility testing of sterile drugs, components, and devices. |
Optimized for aseptic filling and repackaging of sterile components, compounding, preparation of medical drugs and devices, and more. |
A modular isolator system provides flexible configuration and modification possibilities to meet your specific process and application requirements. |
|
ISOTEST Isolators |
||
Learn more about Sterile Isolation Technology
From cleanrooms to laminar flow hoods, biological safety cabinets, isolators, and restricted access barrier systems, numerous innovations protect the process, the operator, and the surrounding environment.
Get the full comparison and learn more about improving contamination control by downloading the white paper: Comparing Laminar Flow Hood and Barrier Technology in Aseptic Processing.
