ECMO and the
COVID-19 pandemic
The COVID-19 pandemic created unique challenges to test whether extracorporeal membrane oxygenation (ECMO) can increase the survival of critically ill patients with severe acute respiratory distress syndrome (ARDS). Emerging research shows a mortality benefit for COVID-19 patients with ARDS who are supported with ECMO. However, as healthcare resources are still very limited and there is a lack of consensus on patient selection across medical centers, only a small proportion of patients benefited from this potentially lifesaving strategy. Based on some lessons learned, we now know more about which types of patients benefit most and under which conditions.
The role of VV-ECMO in treating severe ARDS
ARDS is a critical respiratory condition where damaged lungs are unable to adequately transfer oxygen to blood, causing a dangerous drop in blood oxygen content that compromises the function of other organs. Mechanical ventilation can help many ARDS patients. However, in severe cases where lung compliance decreases dramatically, pressure-positive ventilation perpetuates the inflammatory insult, which is associated with several complications and poor clinical outcomes. Prior to the COVID-19 pandemic, it was noted that VV-ECMO (veno-venous extracorporeal membrane oxygenation) reduced mortality in patients who developed progressive, acute respiratory failure despite optimal support from conventional mechanical ventilation. Applying VV-ECMO maintains gas exchange and mitigates potential damage caused by mechanical ventilation, allowing the lungs to recover [4][5][6].
Approximately 40% of COVID-19 patients admitted to Intensive Care Units (ICU) develop severe ARDS [1][2], making it the most common life-threatening condition associated with a SARS-CoV-2 infection [3]. The COVID-19 pandemic created new ways to demonstrate the effectiveness of VV-ECMO as a bridging strategy to gain valuable time, both for recovery and for the application of additional patient-specific therapies and treatment.
COVID-19 associated ARDS vs. ARDS of other causes:
Any impact on ECMO outcomes?
VV-ECMO was used early during the pandemic to treat the most severe forms of COVID-19 associated ARDS [7]. While some initial reports indicated high mortality [8], subsequent studies showed significantly more positive outcomes. Not surprisingly, ECMO results for ARDS caused by COVID-19 and other types of ARDS are not substantially different.
For example, the international cohort study conducted by the ELSO (Extracorporeal Life Support Organization) Registry showed an estimated cumulative hospital mortality of 37% for critically ill adults 90 days after initiation of ECMO, when conducted at experienced centers [9]. Based on 1.035 ECMO patients from 213 centers in 36 countries, this report sends an important message and allows for a general estimate of mortality in the context of COVID-19 infections supported with ECMO for the first time. Another retrospective observational study shows that for those patients who were considered not suitable for ECMO, the 90-day mortality rate was as high as 86%, comparing with patients who received ECMO support immediately or after optimization of the medical treatment, where mortality rates were 49% and 46%, respectively [20]. Other studies have reported comparable outcomes to previous observations [10][11][12][13] with regards to the effect of ECMO in patients with other causes of acute respiratory failure [4][5], although mortality rates partly vary over the course of the pandemic and in different countries [14][15][16][17][18].
| COVID-19 associated ARDS |
ARDS of other causes | ||||||
| Country | Chile [13] | France [11] | USA [12] | International [9] | International [10] | International [4] | UK [5] |
| Study type | Cohort study | Retrospective cohort study | Multi-center study | Cohort study | Meta-analysis | Multi-center study | Multi-center study |
| Patients | 85 | 83 | 190 | 1.035 | 1.896 | 249 | 180 |
| 60d mortality | 38% | 31% | 33% | 37% | 36% | 40% | 37% |
Table 1: A study comparison between Covid-19 associated ARDS and ARDS of other causes
This overview shows the mortality rates of patients with COVID-19 associated ARDS across different countries. ECMO results do not drastically differ between COVID-19 and other causes of acute respiratory distress.

The effect of ECMO availability on patient survival
During the COVID-19 pandemic, the number of patients with severe ARDS exceeded the capacity of healthcare facilities to provide ECMO therapy. In a prospective study from 2022, Gannon et al. [15] examined whether a healthcare system’s capacity to provide ECMO had an impact on the survival of patients with severe ARDS due to COVID-19. The authors analyzed consecutive patients with severe ARDS from a SARS-CoV-2 infection, who were referred for ECMO to a single center during an eight-month period in 2021. 90 patients were medically eligible to receive ECMO and were included in the study. 35 out of 90 patients received ECMO, while the remaining 55 patients did not receive ECMO due to the health system’s limited capacity to provide the therapy. As a result, 89.1% of patients who did not receive ECMO died before hospital discharge, compared to 42.9% of those who did receive ECMO (see Graph 1). Both groups were similar in terms of demographics, comorbidities, and ventilation settings.
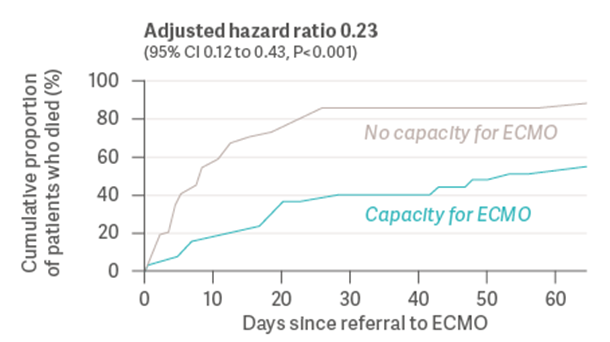
Graph 1: Cumulative proportion of patients who died before hospital discharge (Gannon et al., 2022) [15]
Although these results lacked randomization and had several other limitations, the Gannon study raises an important question: May the inability to provide ECMO to all eligible patients due to limited healthcare system resources have resulted in preventable deaths from ARDS?
Proven, lower mortality
In another large observational study from 2022, Urner et al. [19] quantified the effect of ECMO and its potential survival benefit. The study examined data from the COVID-19 Critical Care Consortium registry, including 7.345 adult patients from 30 countries. These patients were admitted to the ICU with a SARS-CoV-2 infection between January 2020 and August 2021. The study showed that mortality rates for patients receiving ECMO were 7.1% lower than those without ECMO intervention (see graph 2). Age, severity of hypoxemia, and duration and intensity of mechanical ventilation were found to be modifiers of treatment effectiveness.
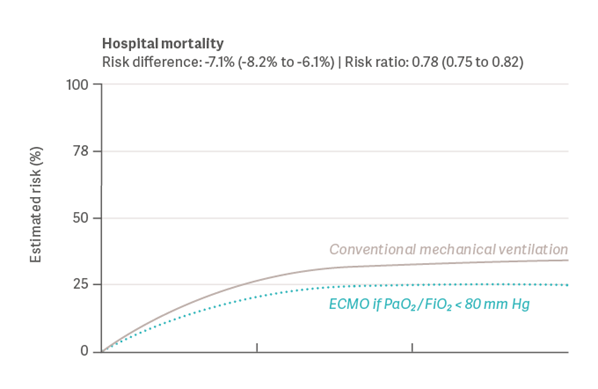
Graph 2: Results for Hospital Mortality (Urner et al., 2022) [19]
Again, within the limitations of observational data, the study indicates that ECMO would have improved outcomes if consistently provided to well-selected patients with more severe hypoxemia, or with exposure to higher intensities of mechanical ventilation.
What Have We Learned?
In patients with severe ARDS, ECMO has already in the past been proven to work and deliver lower mortality rates [4]. Likewise, recent data from the COVID-19 pandemic strongly indicate that even in stressful circumstances, COVID-19-associated ARDS can be efficiently treated with ECMO [3]. The available data seem to strongly support a potential mortality benefit, specifically for selected, severely ill patients [17].
Current evidence shows that certain factors increase the survival probability of patients, with VV-ECMO as a potential rescue procedure for COVID-19-associated ARDS. A younger age [3][9][16][17][18][19] and a rapid decision to initiate ECMO within 7 days [3][16] are independently associated with improved survival, as is performing ECMO in experienced centers with at least 30 cases per year [3] - a phenomenon that has also become evident in many other clinical scenarios [23]. The severity of hypoxemia [9][19], the intensity [19] and duration of mechanical ventilation [16][19] as well as optimal ARDS therapy before ECMO with the administration of neuromuscular blockers and prone positioning [3][9][16] proved to be additional influencing factors for the effectiveness of treatment. Comorbidities such as advanced cardiac, respiratory, or liver failure, irreversible organ damage and renal impairment prior to ECMO seem to be risk factors for bad clinical outcomes [3][9].
The individual, patient-centric decision to implement and manage ECMO correctly in patients with severe ARDS naturally remains complex, especially in the face of limited resources during a pandemic. The search for the sweet spot, or the "effective zone" in which ECMO can achieve its optimum effect has long been a concern for experts. "Don't blame the tool" comments Alain Vuylsteke from NHS Royal Papworth Hospital in Lancet [22]: "Selecting the right patient at the right time is based on clinical principles and experience, as well as availability. Clinicians carefully and subjectively weigh up the risks and benefits of starting ECMO for each patient. The turning point at which one is better than the other is unknown, even if the criteria have been well defined and are regularly reviewed [21]."
Therefore, experience with corresponding case quantity is a decisive factor. Special inclusion and exclusion criteria as well as a standardization of treatment regimens could be the key to smart patient selection [18]. Following expert consensus on this area [24] may help other centers to move forward in the implementation of this valuable therapeutic strategy. Experience is also a way of acquiring knowledge, and knowledge can be quantified, digitalized and shared nowadays at a very quick pace. Perhaps technology can help unexperienced centers to improve decision-making in patient selection in the near future.