Cardiogenic Shock
Timing is everything

It can happen anywhere[1]


Cardiogenic shock patients represent a wide spectrum of disease that requires tailored therapy to improve hemodynamic derangements[2]
Develop a treatment plan
Identify
Any attempt to improve outcomes in CS should begin with its early identification. Models of care including a multi-disciplinary CS team, hold potential for the early identification and individualized treatment of CS.[3]
Evaluate
Quick feedback loops incorporating patient status and hemodynamics are required to assess the need for response to initial therapies.[2]
Escalate
When patients do not respond to treatments initiated, consider the next level of support and transfer to experienced shock centers if required.[4]
Identification is critical
Stages of Cardiogenic Shock [4]
At Risk: A patient with risk factors for cardiogenic shock who is not currently experiencing signs or symptoms. For example, large acute myocardial infarction, prior infarction, acute and/ or acute on chronic heart failure.
Beginning: A patient who has clinical evidence of relative hypotension or tachycardia without hypoperfusion.
Classic: A patient presenting with hypoperfusion requiring intervention beyond volume resuscitation(inotrope, pressor, or mechanical support). These patients typically present with relative hypotension.
Deteriorating: A patient who fails to respond to initial interventions. Similar to Stage C and getting worse.
Extremis: A patient being supported by multiple interventions who may be experiencing cardiac arrest with ongoing CPR.
Any attempt to improve outcomes in cardiogenic shock should begin with early identification. Models of care including a multi-disciplinary CS team hold potential for early identification and individualized treatment.[3]
Initiate early
Retrospective analysis indicates early use of mechanical circulatory support (MCS) is an important therapeutic intervention. Early use of intra-aortic balloon counterpulsation is associated with survival benefits, regardless of the etiology. [5]
30-day survival was 76% when IABP was placed within < 1 hour of onset of cardiogenic shock.[5]
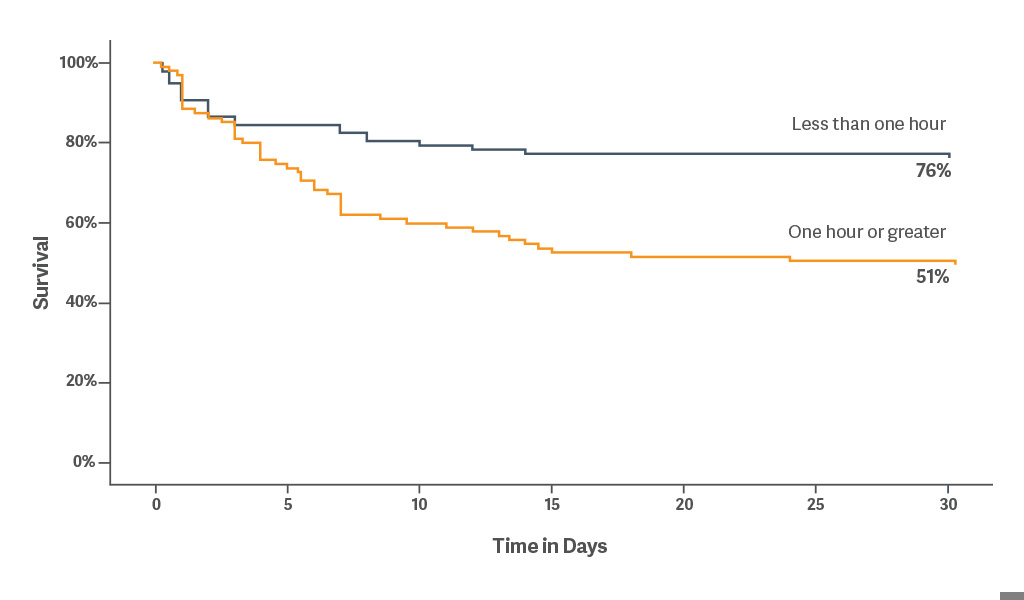
Early initiation of IABP may provide hemodynamic benefit as primary treatment for advanced decompensated heart failure.[6]
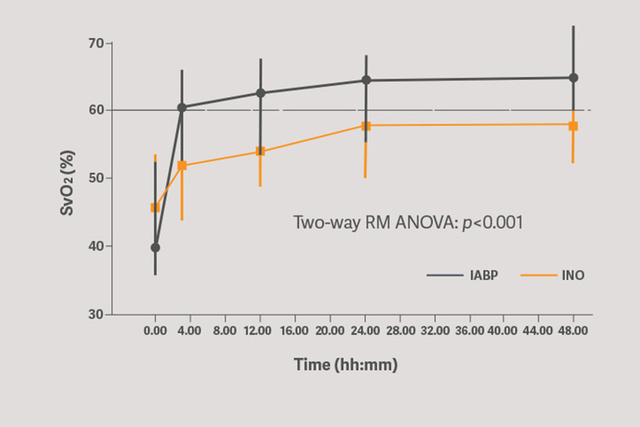
Primary circulatory support with the Sensation Plus 50 cc IABP showed a significant increase in improved organ perfusion assessed by SVO2.[6]
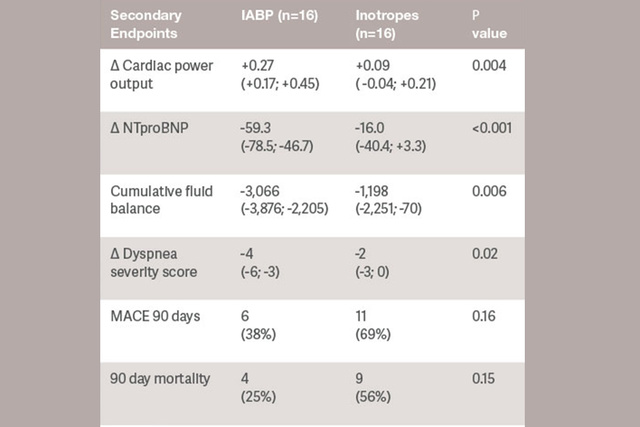
Starting support immediately reduces stroke work, possibly decreasing myocardial oxygen consumption. IABP counterpulsation decreases LV afterload, preload and intraventricular dyssynchrony.[6]
Evaluate effectiveness
Tailor the care to the patient and escalate as needed. Evaluating the response to therapy is critical in making adjustments to the plan of care.[2]
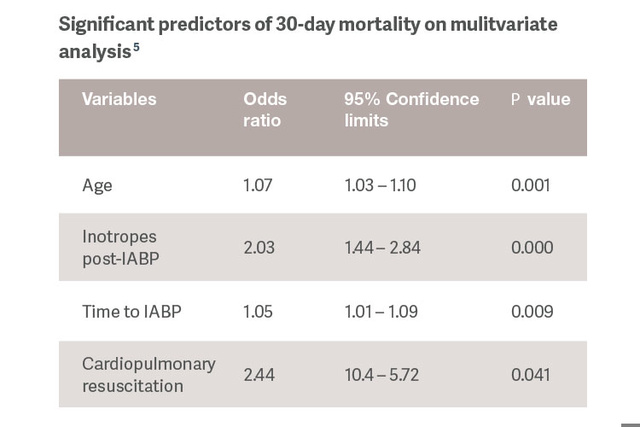
Identification of predictors of response to IABP support would allow us to tailor therapy and reserve use of more powerful MCS devices for patients that have more advanced stages of CS.[5]
IABP: the safe first-line MCS option
| Article | Number of patients | Mortality | Bleeding | Stroke | Vascular complications |
AKI |
| Dhruva 2019[8] | 1680 Matched pairs from NCDR* |
Favors IAB Absolute difference 10.9% |
Favors IAB Absolute difference 15.4% |
NA | NA | NA |
| Amin 2019[9] | 48,306 Premier database* |
Favors IAB p < 0.0001 |
Favors IAB p = 0.045 |
Favors IAB p < 0.0001 |
NA | Favors IAB p = 0.052 |
| Wernly 2019[10] | 588 Meta-analysis from 4 RCT** |
No difference p = 0.38 |
Favors IAB p = 0.002 |
No difference p = 1.00 |
Favors IAB p = 0.01 |
NA |
| Schrage 2011[11] | 237 Matched pairs from IABP-Shock II** |
No difference p = 0.64 |
Favors IAB p < 0.01 |
NA | Favors IAB p = 0.01 |
NA |
*Impella vs. IABP
**Impella vs. control (IABP and/or medical treatment)
Complications matter
Trial enrollment: CRISP AMI, n = 337; SHOCK II, n = 600