PERFECTION Trial Overview
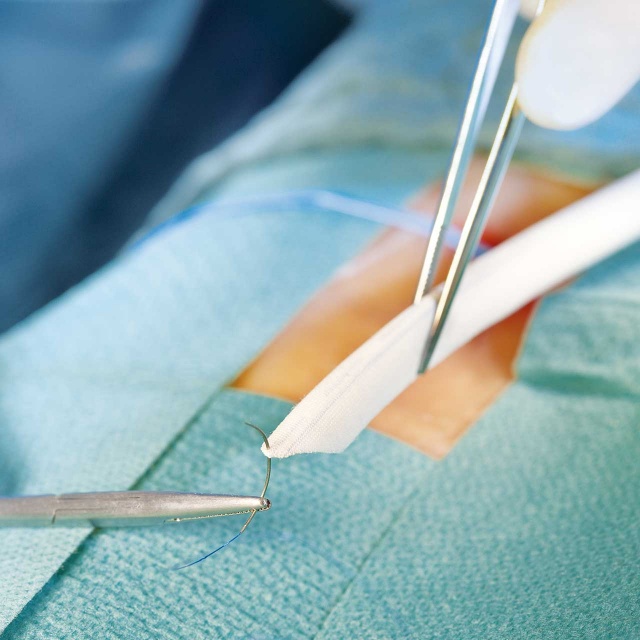
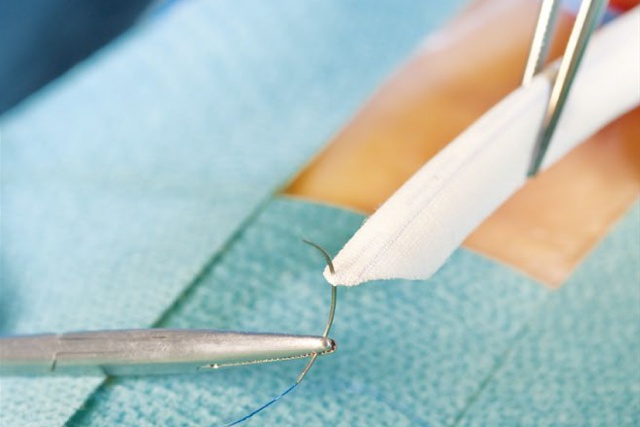
The PERFECTION trial was a prospective, multi-center study designed to assess the clinical efficacy of the novel Fusion vascular graft.[3]
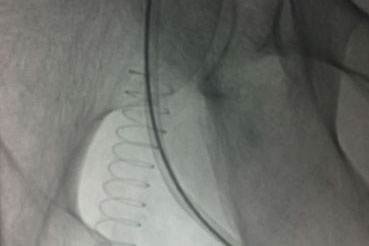
- 117 patients underwent femoral-popliteal bypass with the Fusion vascular graft
- Duplex ultrasound imaging and ankle-brachial indices were performed at 30 days, 6 months and 12 months
- The primary efficacy end point was 12-month primary patency
Results
- The 30-day primary graft patency was 95.3%
- The 12-month primary rate was 85.6%, and the secondary patency rate was 93.2%
- Ankle-brachial indices increased from a mean of 0.53 +/- 0.20 preoperatively to 0.97 +/- 0.16 at 30 days and 0.91 +/- 0.22 at 12 months
Patency rates equaled or exceed those reported with other nonbioactive vascular grafts and compare favorably with data from many studies of heparin-coated prostheses.
Conclusion
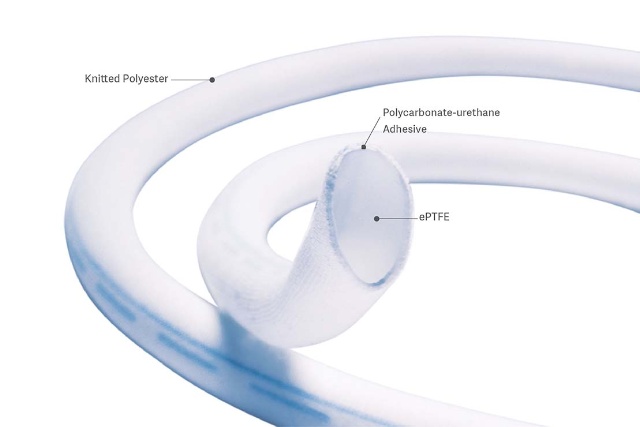
The PERFECTION trial concluded that the Fusion vascular graft is an effective alternative to ePTFE grafts and should be considered as an option in patients requiring femoral-popliteal arterial reconstruction.
Straight |
 |
| Diameter | Length | Reference |
| 5 mm | 40 cm | M002015010450 |
| 5 mm | 80 cm | M002015010850 |
| 6 mm | 20 cm | M002015010260 |
| 6 mm | 40 cm | M002015010460 |
| 6 mm | 60 cm | M002015010660 |
| 6 mm | 80 cm | M002015010860 |
| 7 mm | 40 cm | M002015010470 |
| 7 mm | 80 cm | M002015010870 |
| 8 mm | 40 cm | M002015010480 |
| 8 mm | 60 cm | M002015010680 |
| 8 mm | 80 cm | M002015010880 |
| 10 mm | 40 cm | M002015010410 |
| 10 mm | 80 cm | M002015010810 |
Helix supported |
 |
| Diameter | Length | Reference |
| 5 mm | 40 cm | M002015030450 |
| 5 mm | 80 cm | M002015030850 |
| 6 mm | 40 cm | M002015030460 |
| 6 mm | 60 cm | M002015030660 |
| 6 mm | 80 cm | M002015030860 |
| 7 mm | 40 cm | M002015030470 |
| 7 mm | 80 cm | M002015030870 |
| 8 mm | 40 cm | M002015030480 |
| 8 mm | 60 cm | M002015030680 |
| 8 mm | 80 cm | M002015030880 |
| 10 mm | 40 cm | M002015030410 |
| 10 mm | 80 cm | M002015030810 |
All grafts are fully supported except 3 cm on 1 end.