Best Practices for Multi-Use Bioreactor Sterilization
Regardless of whether the bioreactor is being prepared for a same-use application or an entirely different one, all bioreactor components, as well as liquid media, must be made ready through an effective and reproducible process.
Sterilization Process
Regardless of operation mode, the autoclave must meet the minimum temperature plateau of 121°C with a minimum dwell time of 20 minutes to ensure the sterilization cycle’s efficacy.
3 test types and 3 sterilization methods
-
Standard Test Cycle
Steam injection heats to 121°C for a minimum of 20 minutes. -
Pre-Vacuum
Pre-vacuum is added to increase air removal and improve steam penetration within vessel and tubing. -
Pre-Vacuum and Pressure Pulses
Pre-vacuum is followed by steam pressure pulses to remove any remaining air and provide maximum steam penetration in vessel and tubing.
Empty and Full Bioreactor
To evaluate the efficacy of an extended dwell time a test was performed in Getinge Sterilization Test Lab.
Testing compared the results of a 40-minute dwell time in an empty bioreactor (CC 899) with a 3L vessel filled 2.4L (CC 900) for the same dwell time.
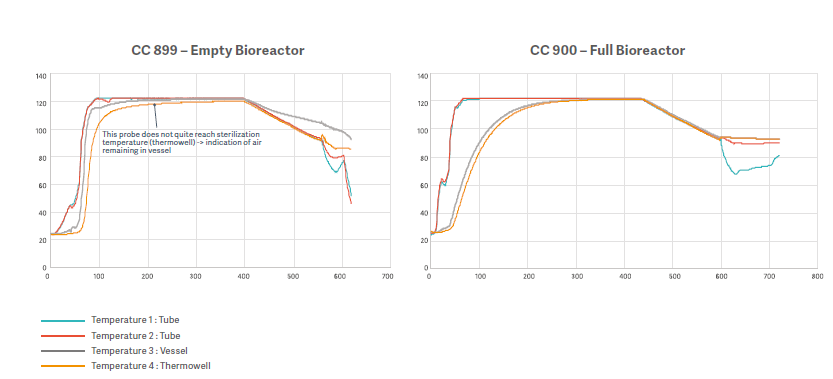
In CC 899, the temperature sensor placed in the thermowell still did not reach 121°C, even by the end of the 40-minute period. However, bioindicator test results indicated no bacterial growth, verifying that extended plateau time improves sterilization results.
In CC 900, the extended plateau time and reduced liquid in the vessel allowed the minimum temperature to be reached throughout. Bioindicator results also verified sterilization in this scenario.
In CC 900, the extended plateau time and reduced liquid in the vessel allowed the minimum temperature to be reached throughout. Bioindicator results also verified sterilization in this scenario.

